Paris, 25 May 2016 – GenSight Biologics, a biotechnology company discovering and developing novel gene therapies for neurodegenerative retinal diseases and diseases in the central nervous system, announces the registration of its document de base with the French Autorité des Marchés Financiers (AMF) under number I.16-049 on May 24, 2016 in relation to its planned IPO on Euronext’s regulated market in Paris.
The registration of the document de base is the first step in GenSight Biologics’ planned IPO on Euronext’s regulated market in Paris, which is contingent on market conditions and regulatory requirements including the AMF’s visa on a prospectus to be prepared as part of the transaction.
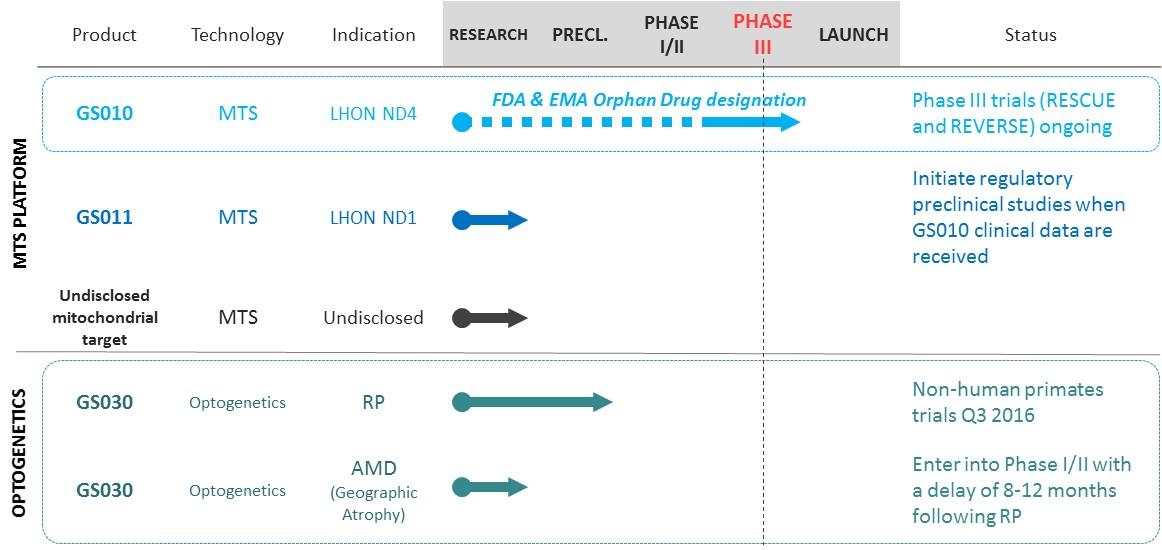
GenSight Biologics is developing two core technology platforms: Mitochondrial Targeting Sequence (MTS), and optogenetics. Out of a large number of applications, GenSight Biologics has chosen to initially focus on neurodegenerative retinal diseases. GenSight Biologics’ product candidates GS010 and GS030 are designed to be administered in a single treatment to each eye by intravitreal injection, in order to offer patients a long-lasting functional cure.
GenSight Biologics’ most advanced lead product candidate GS010 targets Leber Hereditary Optic Neuropathy (LHON) and is currently in Phase III. GS010 has received Orphan Drug designation in Europe and the United States. GenSight Biologics’ MTS technology platform was originally developed by the teams of Pr. José Sahel at the Institut de la Vision in Paris and is protected by patents over which the company has acquired exclusive licenses in ophthalmology and non-exclusive licenses in other mitochondrial diseases. GenSight Biologics believes that its MTS technology platform can be used to address indications outside of ophthalmology involving defects of the mitochondrion.
GenSight Biologics’ second product candidate, GS030, based on the optogenetics technology, is currently at the preclinical stage. The optogenetics technology confers light sensitivity to neurons, which allows to specifically stimulate targeted cells without affecting neighboring cells.GS030 is initially intended to treat all forms of genetic retinitis pigmentosa, and will subsequently target geographic atrophies, resulting from the late-stage form of age-related macular degeneration. GenSight Biologics is expected to initiate a Phase I/II clinical trial with GS030 during the second half of 2017, subject to the requirements of regulatory agencies.
“The IPO project is a significant new step in GenSight Biologics’ development. Our lead product candidate GS010, currently in Phase III clinical trials, could be two years away from filing a regulatory approval for a marketing authorization from 2018 onwards. If the results from the ongoing clinical trials meet expectations, we could offer patients a treatment that should allow them to gain autonomy and benefit from an improved quality of life.” commented Bernard Gilly, CEO of GenSight Biologics.
6 million patients suffer from blindness in Europe and North America. Based on data from regional studies, GenSight Biologics estimates the incidence of LHON to be approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe. RP is the most widespread hereditary cause of blindness in developed nations, with a prevalence of about 1.5 million people throughout the world.
Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease resulting in sudden irreversible vision loss for adolescents and young adults
LHON, targeted by GS010, is a rare mitochondrial genetic disease characterized by the degeneration of retinal ganglion cells that results in rapid, severe and irreversible vision loss, that can lead to complete blindness, and mainly affects adolescents and young adults. LHON alters the patients’ ability to perform daily life activities, such as reading, driving, or facial recognition. Patients’ autonomy is generally very limited, with a significant impact on their families, and substantial direct and indirect costs related to the management of this disability.
GS010: Promising clinical results…
In April 2015, GenSight Biologics completed the recruitment of 15 patients for its Phase I/II study to evaluate the safety and tolerability of GS010. The preliminary results on humans have demonstrated a good safety and tolerability profile.
Patients will be closely followed for a minimum of 3 years. Preliminary pharmacodynamics results at 48 weeks have demonstrated improvements in visual acuity and color perception for treated eyes, in patients with an onset of disease of less than 2 years. Improvements in visual field and color perception have also been observed in patients with the best preserved function before treatment.
Although the study was not designed to demonstrate efficacy of the treatment, due to the diversity of doses and the heterogeneity of patients, these preliminary results are encouraging. After talks with experts, GenSight Biologics has designed its ongoing Phase III trials to target a more homogeneous patient population, very recently diagnosed (less than 12 months), which could maximize the benefits and efficacy of treatment.
… that have enabled the launch of the Phase III trials in Europe and the US at the end of 2015
GenSight Biologics initiated two Phase III trials in late 2015 simultaneously in Europe and the US, to demonstrate the efficacy of GS010 in LHON patients who have suffered a loss of visual acuity with an onset of less than a year.
The main objective of these studies, named “RESCUE” and “REVERSE”, is to assess the efficacy of GS010 in the treated eye versus the untreated eye, by measuring the change in visual acuity at 48 weeks compared to the visual acuity at baseline.
The 36 patients in each study will be randomized in 7 specialized investigation centers (one in France, one in Germany, one in Italy, one in the UK and 3 in the United States).
GS030: optogenetics for the treatment of retinitis pigmentosa and advanced forms of age related macular degeneration
Photoreceptor degeneration can be of genetic origin – in retinitis pigmentosa (RP) – or be linked to aging – in late forms of macular degeneration – but inevitably leads to blindness. Optogenetics uses gene therapy to transform other cells of the retina into photoreceptor cells aiming to restore vision in all patients, regardless of the origin of the disease. Gensight Biologics believes that its GS030 product candidate would benefit patients in the early stages of RP.
A Phase I / II clinical trial for GS030 for the treatment of all forms of retinitis pigmentosa is expected to be initiated during the second half of 2017, subject to the requirements of regulatory agencies. In addition to confirming GS030’s tolerability profile, the trial will yield its first pharmacodynamic results in the first half of 2018. In parallel, GenSight Biologics is also developing GS030 for the treatment of geographic atrophies resulting from the late-stage form of age-related macular degeneration, and is expected to initiate a Phase I / II clinical trial, within 8 to 12 months following the results obtained from retinitis pigmentosa patients.
How to obtain the registration document – GenSight Biologics’ registration document is available upon request and free of charge from GenSight Biologics (74 rue du Faubourg Saint-Antoine, 75012 Paris, France), or on the company’s website (www.gensight-corp.com) and the AMF’s website (www.amf-france.org).
Risk factors – GenSight Biologics draws the public’s attention to chapter 4 “Risk factors” of the registration document and particularly to the risk factors set out in section 4.1 “Risks relating to the Group’s products, market and business”.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biotechnology company discovering and developing novel therapies for neurodegenerative retinal diseases and diseases of the central nervous system. GenSight Biologics’ pipeline leverages two core technology platforms, Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from severe degenerative retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber’s Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible vision loss in teens and young adults. Using its gene therapy-based approach,
GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection in order to offer patients a sustainable functional visual recovery.
Disclaimer
This press release and the information contained herein do not constitute either an offer to sell or purchase, or the solicitation of an offer to sell or purchase, securities of GenSight Biologics S.A. (the “Company”).
No communication and no information in respect of the offering by the Company of its shares may be distributed to the public in any jurisdiction where registration or approval is required. No steps have been taken or will be taken in any jurisdiction outside France where such steps would be required. The offering or subscription of shares may be subject to specific legal or regulatory restrictions in certain jurisdictions. The Company takes no responsibility for any violation of any such restrictions by any person.
This announcement does not, and shall not, in any circumstances, constitute a public offering nor an invitation to the public in connection with any offer. The distribution of this press release may be restricted by law in certain jurisdictions. Persons into whose possession this press release comes are required to inform themselves about and to observe any such restrictions.
This announcement is an advertisement and not a prospectus within the meaning of the Prospectus Directive (as defined below), as implemented in each member State of the European Economic Area.
With respect to the Member States of the European Economic Area other than France (“Member States”), no action has been undertaken or will be undertaken to make an offer to the public of the securities referred to herein requiring a publication of a prospectus in any Member State. As a result, the securities of the Company may not and will not be offered in any Member State except in accordance with the exemptions set forth in Article 3 of the Prospectus Directive.
For the purposes of the provision above, the expression “offer to the public” in relation to any shares of the Company in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase any securities, as the same may be varied in that Member State. The expression “Prospectus Directive” means Directive 2003/71/EC (as amended, including by Directive 2010/73/EU), and includes any relevant implementing measure in the Member State.
This press release may not be distributed, directly or indirectly, in or into the United States. This press release does not constitute an offer of securities for sale nor the solicitation of an offer to purchase securities in the United States or any other jurisdiction where such offer may be restricted. Securities may not be offered or sold in the United States absent registration under the U.S. Securities Act of 1933, as amended (the “Securities Act”) except pursuant to an exemption from, or in a transaction not subject to, the registration requirements thereof. The securities of the Company have not been and will not be registered under the Securities Act, and the Company does not intend to make a public offer of its securities in the United States. Copies of this press release are not being, and should not be distributed in or sent into the United States.
The distribution of this press release (which term shall include any form of communication) is restricted pursuant to Article 21 (restrictions on financial promotion) of Financial Services and Markets Act 2000 (“FMSA”). This press release is directed only at persons who (i) have professional experience in matters relating to investments and fall within Article 19(5) (“investment professionals”) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005, or (ii) are persons falling within Article 49(2)(a) to (d) (“high net worth companies, unincorporated associations etc.”) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 or (iii) are persons to whom this communication may otherwise lawfully be communicated (all such persons in (i), (ii) and (iii) above together being referred to as “Relevant Persons”). This press release must not be acted on or relied on in the United Kingdom by persons who are not Relevant Persons. Any investment or investment activity to which this press release relates is available only in the United Kingdom to Relevant Persons, and will be engaged in only with such persons. Any person other than a Relevant Person may not act or rely on this press release or any provision thereof. Persons distributing this press release must satisfy themselves that it is lawful to do so.
A Prospectus is been prepared (consisting of (i) a document de base and (ii) a note d’opération including the summary of the Prospectus) and will receive visa from the AMF. This Prospectus includes a section describing certain risk factors relating to the company and the offering. This Prospectus will be available on the AMF web site (www.amf – france.org) and on the company’s web site (www.gensight-biologics.com). Potential investors should review the risk factors described in the Prospectus.
This press release may not be distributed, directly or indirectly, in or into the United States, Canada, Australia or Japan.
Contacts
-
GenSight BiologicsChief Financial OfficerThomas Gidoin+33 (0) 6 01 36 35 43