Clinical development summary
Our pipeline currently consists of:
- Two lead product candidates for the treatment of sight-threatening retinal degenerative diseases, together with
- Products in preclinical development targeting ophthalmic and neurodegenerative diseases
Visit www.clinicaltrials.gov and search for “GenSight Biologics” to learn more about our ongoing clinical trials listed below.
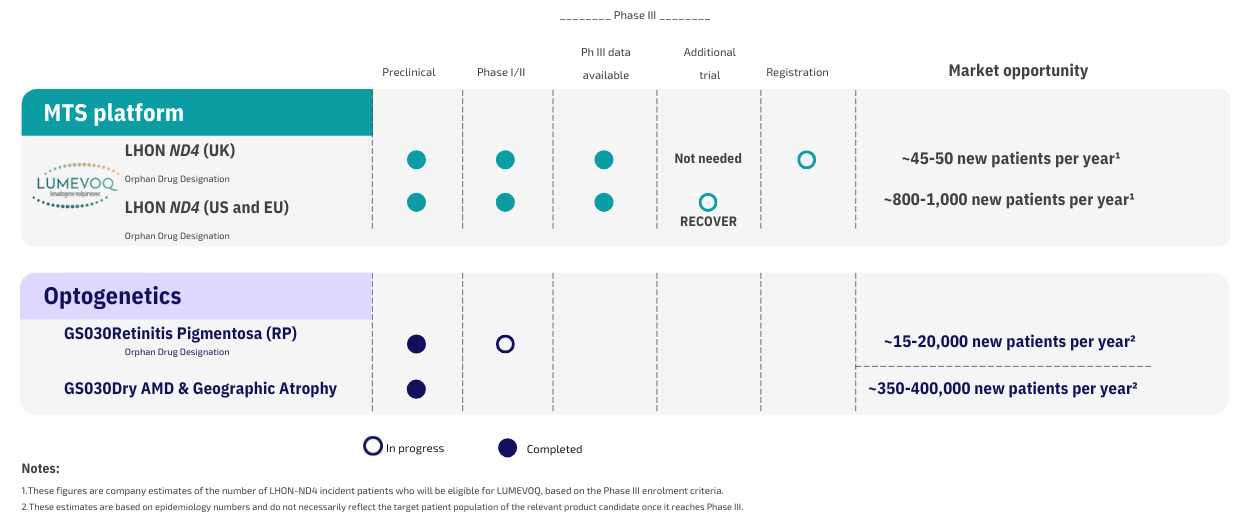
Our products
-
GS010 (lenadogene nolparvovec)
GS010 is an AAV2 gene therapy vector that encodes the human wild-type ND4 protein.
The ND4 gene is normally located in the mitochondria where ND4 proteins are synthesized. GS010 allows allotopic expression of the mitochondrial gene ND4 in the nucleus thanks to a proprietary Mitochondrial Targeting Sequence that shuttles the messenger RNA from the nucleus directly to the outer membrane of the mitochondria. There, the ND4 proteins are synthesized and incorporated into the mitochondria. Wild-type ND4 proteins then integrate into Complex I of the respiratory chain and rescue the deficiency.
Trials with enrollment already closed
A Phase I/II trial, completed in France subjects with long-standing vision loss from LHON with the ND4 gene mutation.
Two Phase 3 trials (RESCUE and REVERSE), to evaluate the efficacy of GS010 in LHON patients with a recent onset of disease (i.e., less than one year), were completed in 2019 in Europe and the US. Results of follow-up visits up to Week 96 in both trials were reported in 2019. Patients in both trials were invited to participate in a long-term follow-up study, which will monitor safety and efficacy up to 5 years after injection.
A third Phase 3 trial, is the ongoing REFLECT study, which evaluates the efficacy and safety of bilateral intravitreal injection of GS010 for the treatment of vision loss up to 1 year.
Finally, the company also completed a retrospective registry study REALITY to study the evolution of visual function among untreated LHON patients. This study completed patient recruitment in 2019.
Studies being initiated
There are currently no trials involving GS010 that are enrolling patients.
Expanded access / compassionate use
For any request regarding Early Access Program please use the contact form: Contact Us – GenSight Biologics (gensight-biologics.com)
-
GS030 (Gene Therapy + Optogenetics)
GS030 is an innovative combination of two complementary components
- A gene therapy product encoding a photoactivatable channelrhodopsin protein, delivered via a modified AAV2 vector known as AAV2 7m8; and
- Biomimetic goggles that stimulate the engineered retinal cells. Images are projected onto the retina by a light source that uses a specific wavelength.
GS030 uses optogenetics, a biologic technique that involves the transfer of a gene that encodes for a light-sensitive protein, which in turn causes neuronal cells to respond to light stimulation. GS030 includes a bio-engineered AAV2 gene therapy vector that introduces the gene of a photosensitive protein (to which we have exclusive rights in optogenetics) into the nucleus of the target cells, in our case the retinal ganglion cells, or RGCs. This gene confers a photoreceptive function to healthy and preserved RGCs, independent of any specific underlying genetic mutation that caused the photoreceptor degeneration.
However, because cells expressing optogenetic proteins are less light sensitive than normal photoreceptors, vision under regular daylight conditions is unlikely to be possible without further intervention. GS030 also features biomimetic goggles, which mimic the normal retinal activity by capturing visual information and amplifying the light stimulation upon the transduced neuronal cells at the appropriate wavelength.
Studies being initiated
We have initiated the PIONEER study, a dose-escalation study to evaluate the safety and tolerability of GS030 in subjects with Retinitis Pigmentosa.
Expanded access / Compassionate use
For any request regarding Early Access Program please use the contact form: Contact Us – GenSight Biologics (gensight-biologics.com)
-
GS030 (Geographic Atrophy in Dry-AMD)
GS030 is an innovative combination of two complementary components
- A gene therapy product encoding a photoactivatable channelrhodopsin protein, delivered via a modified AAV2 vector known as AAV2 7m8;
and
- Biomimetic goggles that stimulate the engineered retinal cells. Images are projected onto the retina by a light source that uses a specific wavelength.
GS030 uses optogenetics, a biologic technique that involves the transfer of a gene that encodes for a light-sensitive protein, which in turn causes neuronal cells to respond to light stimulation. GS030 includes a bio-engineered AAV2 gene therapy vector that introduces the gene of a photosensitive protein (to which we have exclusive rights in optogenetics) into the nucleus of the target cells, in our case the retinal ganglion cells, or RGCs. This gene confers a photoreceptive function to healthy and preserved RGCs, independent of any specific underlying genetic mutation that caused the photoreceptor degeneration.
However, because cells expressing optogenetic proteins are less light sensitive than normal photoreceptors, vision under regular daylight conditions is unlikely to be possible without further intervention. GS030 also features biomimetic goggles, which mimic the normal retinal activity by capturing visual information and amplifying the light stimulation upon the transduced neuronal cells at the appropriate wavelength.
Clinical trials
We plan to investigating the potential for GS030 to treat GA in Dry-AMD after the PIONEER study has been concluded.
- A gene therapy product encoding a photoactivatable channelrhodopsin protein, delivered via a modified AAV2 vector known as AAV2 7m8;
