- Clinically meaningful improvement of +15 ETDRS letters in visual acuity of treated eyes at Week 72
- Continuous bilateral improvement of both visual acuity and contrast sensitivity
- Significantly higher proportion of drug-treated eyes achieved clinically meaningful improvement in contrast sensitivity
- Sustained preservation of LHON-relevant retinal anatomy in treated eyes further demonstrates the neuroprotective effect of GS010
Paris, France, October 18, 2018, 7.30 am CEST – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported additional results at Week 72 from the REVERSE Phase III clinical trial, which evaluates the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in 37 subjects whose visual loss due to 11778-ND4 Leber Hereditary Optic Neuropathy (LHON) commenced between 6 and 12 months prior to study treatment.
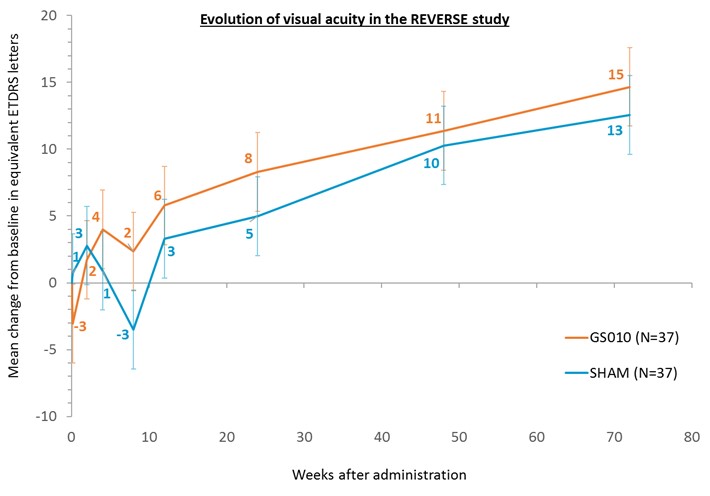
At 72 weeks, a clinically meaningful improvement from baseline in mean visual acuity of +15 letters
(-0.294 LogMAR) was observed in GS010-treated eyes, with concomitant contralateral improvement of +12 letters (-0.246 LogMAR)[1] in sham-treated eyes. This improvement, which extends the positive trend that had been reported at Week 48, points to a sustained functional outcome for the trial subjects.
Continued improvement was also observed in contrast sensitivity as determined by Pelli-Robson low-contrast testing. At 72 weeks, GS010-treated eyes and sham-treated eyes gained on average +0.21 LogCS and +0.15 LogCS versus baseline, respectively. The proportion of treated eyes that achieved a clinically meaningful improvement of at least 0.3 LogCS (45.9%) was statistically significantly higher than that of sham-treated eyes (24.3%; p=0.0047).
The visual function outcomes were accompanied by evidence that GS010 was engaging its anatomic targets, the ganglion cells. At 72 weeks, high-resolution Spectral-Domain Optical Coherence Tomography (SD-OCT) objectively demonstrated sustained preservation of the retina anatomy relevant to LHON in GS010-treated eyes. The ganglion cell layer macular volume was preserved (+0.000 mm3) in treated eyes, while sham-treated eyes deteriorated from baseline (-0.044 mm3). The difference was statistically significant (p=0.0060). Drug-treated eyes also showed a limited loss in thickness of the temporal quadrant of the retinal fiber layer of -1.6 µm, compared to a loss of -3.6 µm in sham-treated eyes (p=0.0521).
“The level of sustained improvement in both visual acuity and low-contrast sensitivity, and the sustained preservation of retinal ganglion cells on OCT, at this stage of disease progression is very encouraging, and is a departure from what has been observed and reported on the natural history of LHON,” commented Dr. Mark L. Moster, Neuro-Ophthalmology, Wills Eye Hospital, Professor of Neurology and Ophthalmology at Thomas Jefferson University, Philadelphia, PA, and Principal Investigator in REVERSE and RESCUE trials.
“We’re seeing visual function continue to improve one and a half years after eyes are treated with GS010, and at the same time, objective tests continue to establish neuroprotection of the retina in treated eyes. This is, beyond any doubt, a great benefit for patients and their families,” commented Bernard Gilly, Co-founder and Chief Executive Officer of GenSight. “These results strengthen our determination to work with regulatory agencies to bring GS010 to market within our defined timelines.”
As per protocol, all 37 subjects will be evaluated again at 96 weeks, and data will be reported in the second quarter of 2019.
Topline 48-week data for RESCUE, the second Phase III clinical trial of GS010 in the treatment of LHON, is expected in early Q1 2019.
[1] A mixed model of repeated measures (MMRM) was used for the analysis of the time course of visual acuity illustrated in the above graph. A mixed model of analysis of covariance (ANCOVA) was used for the analysis of the primary endpoint. Both statistical models used different covariates, accounting for minor differences in the rounding of ETDRS letters.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research works conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled pivotal Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and sham-treated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best seeing eyes that received GS010 compared to those receiving sham, and to worse seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio‑dissemination and the time course of immune response.
The trials are conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Topline results of RESCUE at 48 weeks are expected in early Q1 2019.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767