- Sustained, clinically meaningful bilateral improvement in visual functions 96 weeks after injection
- Observed bilateral improvement interpreted as clearly superior to known natural history
- Improvements in visual function translate to significant increase in quality of life among LHON patients
- GenSight to move GS010 to regulatory approval, first in Europe then in the United States
Paris, France, May 29, 2019, 7:30 am CEST – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported highlights from its recent Key Opinion Leader (KOL) panel hosted in New York City on May 23, 2019. The event was dedicated to updates on clinical results for GS010, in particular findings at Week 96 of the REVERSE Phase III clinical trial in the treatment of Leber Hereditary Optic Neuropathy (LHON).
The panel of medical experts included David J. Calkins, PhD [1]; Sean Donahue, MD, PhD [2]; Mark Moster, MD[3]; José-Alain Sahel, MD[4]; and Robert C. Sergott, MD5. In addition, Andy Marks, LHON patient and patient advocate, spoke about the patient experience in LHON.
Dr. Donahue set up the morning’s discussion by providing an overview of LHON as a genetic disease. Dr. Moster then critically examined the literature on how vision changes after loss, over the natural history of the disease. Dr. Moster highlighted differences in the population studied, in terms of causative mutations, and date of onset in past studies. His analyses, which attempted to account for the factors above, indicate a “huge difference” between the results seen in the REVERSE trial and the outcomes cited in the medical literature.
Just as important, his clinical experience as well as that of his peers are at odds with the sustained improvements observed in REVERSE. “We simply do not see the kinds of improvement in our clinical practices when we follow these patients as have been seen in this trial,” Dr. Moster concluded. He reminded the audience that REVERSE subjects had the most severe mutation in terms of rate of spontaneous recovery.
The visual deterioration has profound effects on patients’ lives, Andy Marks explained. He described having to give up a house that he had just purchased when he experienced visual loss from LHON, and moving his residence closer to his office as he adjusted to a lower level of autonomy. Eventually, he moved from Orlando to the New York City metropolitan area, where his inability to drive would be less decisive for his work life. In the community of LHON patients in which he actively participates, he has yet to encounter someone whose visual functions recovered to the degree observed in the REVERSE trial after disease onset.
“We are living in an age of remarkable gene therapies,” said Dr. Sergott, who reviewed the REVERSE study’s key findings. Among the efficacy findings, he highlighted the recovery of visual acuity – +15 ETDRS letters equivalent improvement versus baseline and +28 ETDRS letters equivalent improvement versus nadir for GS010-treated eyes (+13 and +23, respectively, for sham-treated eyes) – as “extraordinary”.
Dr. Sergott also shared subject-level data, newly available at Week 96, that showed:
- For most patients, eyes tended to track together;
- Most patients improved in both eyes relative to baseline;
- Patients with large improvements from baseline and from nadir tended to have GS010-treated eyes that outperformed their sham-treated eyes; and
- For a greater number of patients, change from nadir is more pronounced in GS010-treated eyes.
Figure 1. Visual Acuity Change from Baseline in LogMAR among REVERSE Subjects
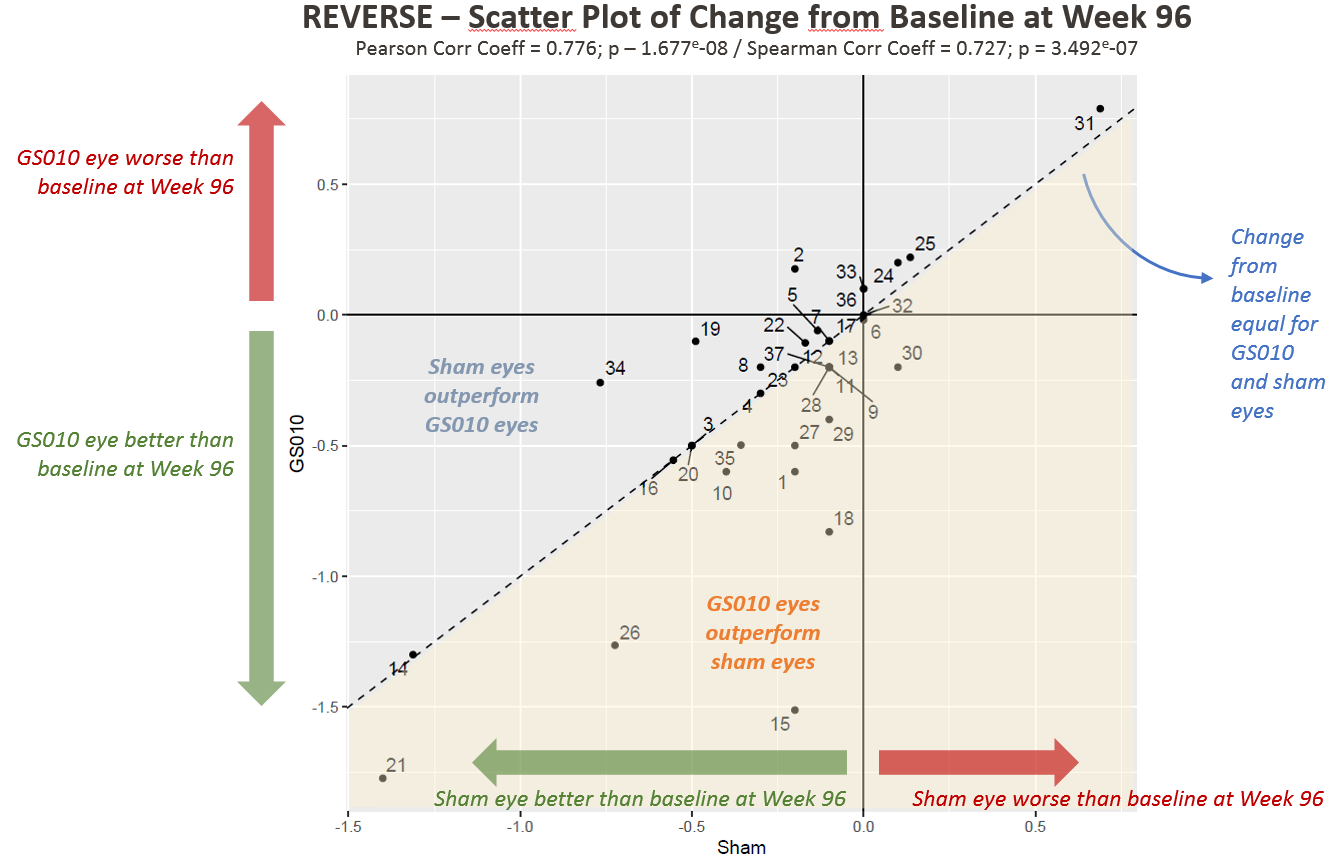
Figure 2. Visual Acuity Change from Nadir in LogMAR among REVERSE Subjects
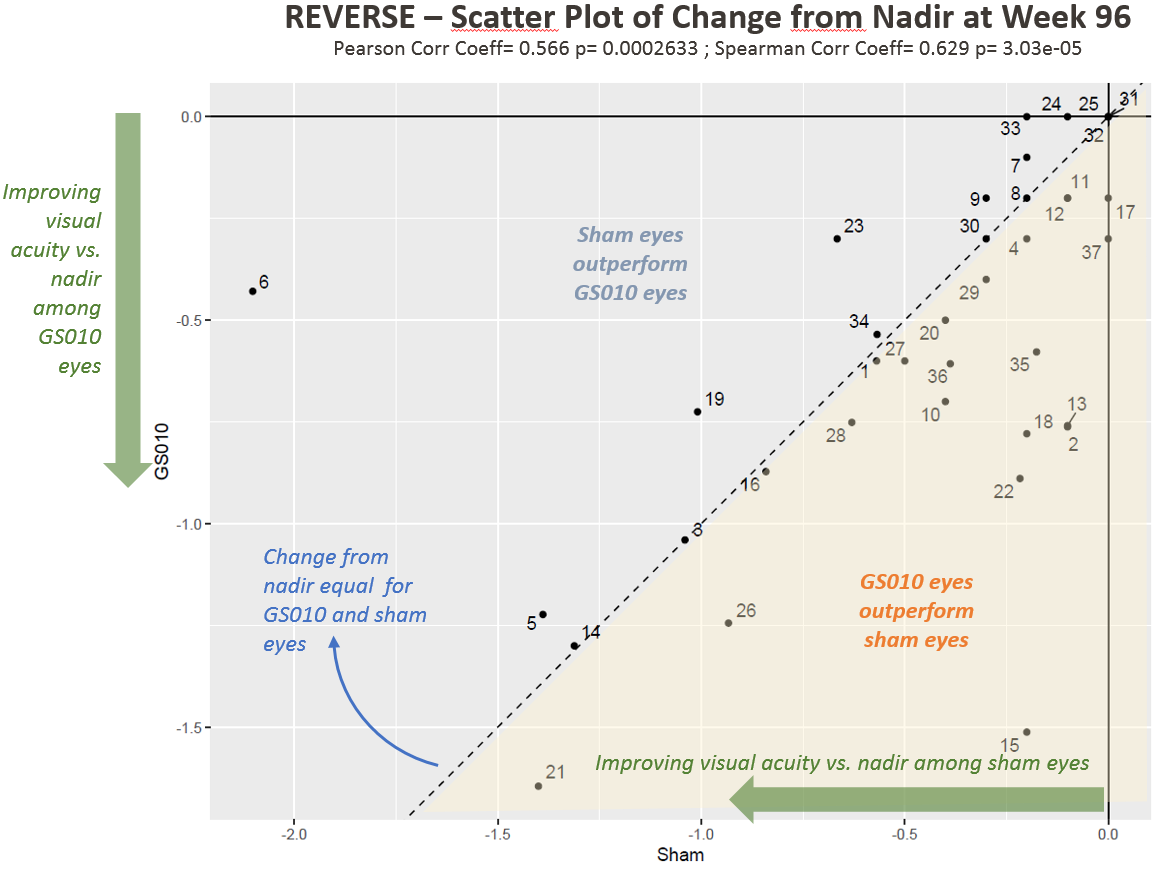
Note: Each point in Figures 1 and 2 represents one patient (two eyes) in REVERSE.
Bilateral improvement was thus a feature of individual data, not just a result of aggregation. An explanation for this durable bilateral improvement, which is at odds with natural history or known clinical practice, was the focus of Dr. Calkins’ talk. He began with the provocative observation that he would have been surprised had there been no effect in the contralateral eye. As he explained, an eye has many ways to interact with and compensate for a weakness in its fellow eye. “The fellow eye ought to improve,” he said.
Dr. Calkins’ team studies how stress in one eye can lead the other (unstressed) eye to share resources via the optic nerve. Dr. Calkins suggested that a likely mechanism for compensation between the eyes lies in the networks formed between astrocyte glia and optic nerve fibers in the retina and optic nerve, which allow sharing of metabolic molecules . “Resources can travel via astrocyte networks from the treated eye to the untreated to improve overall performance,” Dr. Calkins concluded. Recall that GS010 works by restoring the ability of mitochondria in retinal ganglion cells to generate energy needed for visual processing.
Dr. Sahel, co-founder of GenSight, summarized the presentations by reiterating the efficacy findings and rejecting either the “placebo effect” or “training effect” as an explanation for the results. The bilateral evolution of subjects’ eyes would be difficult to reconcile with a “placebo or training effect”, he said. “In which studies would a placebo or training effect show something similar to the initial decrease in an efficacy signal [the nadir effect seen in REVERSE and RESCUE] followed by a sustained increase afterwards? Why would the placebo or training effect work that way? It would not,” he said. “These intriguing results represent good news for patients.”
Dr. Donahue concluded by sharing the experience of a teen-aged patient who was treated with GS010 in a compassionate use program. “He went from needing to be led by his parents around a room to once again being able to play basketball,” he said. “It’s the experience of just one patient, but you can see, it’s very impressive, and the family is very thankful.”
Following the discussion, Bernard Gilly, co-founder and CEO of GenSight, underscored GenSight’s determination to bring GS010 to market as early as possible. “The team is working to prepare the pre-submission meeting with the EMA and the End of Phase 2 meeting with the FDA in order to move GS010 through the regulatory process for approval,” he said.
The presentation is available in replay on the Company’s website at https://www.gensight-biologics.com/2019/05/22/kol-event-to-present-phase-3-reverserescue-results-of-gs010-for-the-treatment-of-leber-hereditary-optic-neuropathy-lhon-new-york-city/.
[1] O’Day Professor, Vice Chair and Director for Research Vanderbilt Eye Institute, Vanderbilt University Medical Center, Nashville, TN
[2] Coleman Professor of Ophthalmology, Neurology and Pediatrics; Vice Chair of Clinical Affairs, Ophthalmology Vanderbilt University Medical Center, Nashville, TN
[3] Neuro-Ophthalmology, Wills Eye Hospital and Professor of Neurology and Ophthalmology at Thomas Jefferson University, Philadelphia, PA
[4] Director of the Institut de la Vision (Sorbonne-Université/Inserm/CNRS), Paris; Chairman of the Department of Ophthalmology at Centre Hospitalier National d’Ophtalmologie des XV-XX, Paris; Professor and Chairman of the Department of Ophthalmology at University of Pittsburgh School of Medicine and UPMC (University of Pittsburgh Medical Center). Co-founder, GenSight.
5 Director, Neuro-Ophthalmology, Wills Eye Hospital; Director, William H. Annesley, Jr, EyeBrain Center, and Professor of Neurology and Ophthalmology at Thomas Jefferson University, Philadelphia, PA
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best‑Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best‑seeing eyes that received GS010 compared to those receiving sham, and to worse‑seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio‑dissemination and the time course of immune response. Readouts for these endpoints are at 48, 72 and 96 weeks after injection.
The trials are conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results are expected in 2019 for both trials, after which patients will be transferred to a long-term follow-up study that will last for three years.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767