- First study to find direct evidence that therapeutic gene is transferred from the injected eye to the uninjected contralateral eye of primates
- Findings support mechanism for bilateral visual improvement with unilateral GS010 gene therapy, which was consistently observed in LHON subjects in the REVERSE and RESCUE Phase III trials
Paris, France, Wednesday, October 9, 2019, 7.30 am CEST – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported positive proof of GS010 DNA transfer from one eye to the other eye following unilateral intravitreal injection of primates. In a non-clinical study to investigate the local biodistribution of GS010, tissue samples from the non-injected eye of monkeys that had been unilaterally injected with GS010 were found to contain GS010 DNA three months after injection, indicating the expression of the therapeutic gene in the contralateral eye
“These results join a growing body of evidence suggesting the two eyes communicate not only in disease, but also in response to treatment,” said David J. Calkins, PhD, O’Day Professor, Vice Chair and Director for Research Vanderbilt Eye Institute, Vanderbilt University Medical Center, Nashville, Tennessee, United States. “With the new understanding these results provide, we can move forward with more precise treatments.”
Performed by CiToxLAB France, a leading CRO for preclinical research, the study was initiated by GenSight to investigate potential mechanisms behind the unexpected contralateral effect seen in two of GS010’s Phase III trials, REVERSE and RESCUE. As previously reported, both trials, which this year completed the two-year follow-up of patients unilaterally injected with GS010, documented sustained bilateral improvements in LogMAR mean visual acuity. The contralateral effect did not conform to expectations for gene therapies administered to only one eye.
The CiToxLAB study uses a purpose-bred species of monkeys, which is favored by scientists and accepted by regulatory bodies due to physiological similarities with humans. For testing at three months, a control monkey was given an intravitreal injection of saline solution in its right eye and was not injected in its left eye. Three test monkeys were given an intravitreal injection of GS010 in their right eyes and not injected in their left eyes. The dosage of GS010 was calibrated to be the allometric equivalent of that used in the GS010 Phase III trials. Three months after the injection, tissues from the right and left eyes were sampled and tested using a qPCR test which had been validated in a dedicated prior study. The highly sensitive and accurate test contains a protocol that specifically targets a portion of the GS010 DNA and can detect the GS010 DNA matrix.
As expected, the qPCR test did not detect the GS010 DNA in any of the tissue samples from the control monkey unilaterally injected with saline solution. Also as expected, the test was able to detect, and in many cases, quantify the presence of GS010 DNA in tissue samples from GS010-injected right eye. Remarkably the qPCR test was also able to detect, and even quantify, viral DNA vector in the contralateral eye, which had received no injection.
Figure 1: Presence of GS010 DNA in the visual and cerebral systems of test monkeys
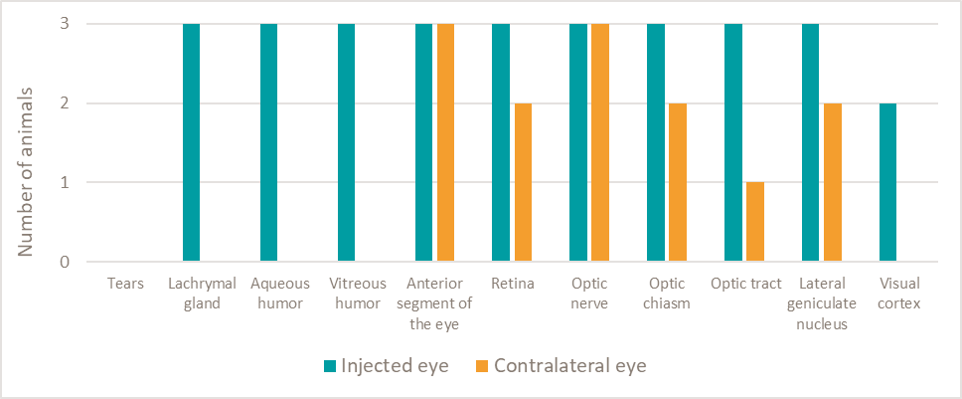
Note: qPCR test used to detect GS010 DNA was validated in a dedicated study conducted prior to the monkey study. The graph depicts the number of monkeys whose tissues contained DNA that were within the sensitivity of the test to detect. In some cases, the levels were above the quantifiable threshold.
DNA was detected and quantified in the anterior segment, the retina, as well as the optic nerve of the non-injected contralateral eye. In addition, DNA was detected and quantified in the optic chiasm, suggesting that the anatomic route taken by the viral vector DNA from the treated eye to the non-treated eye was via the optic nerves and chiasm.
“The identification of viral vector DNA in the contralateral uninjected eye is an important observation with broader relevance to the design of gene therapy trials for optic neuropathies,” noted Dr. Patrick Yu-Wai-Man, Senior Lecturer and Honorary Consultant Ophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, London, United Kingdom. “Although the non-human primate study was not designed to determine the underlying mode of transfer, the presence of viral vector DNA in the optic chiasm and optic nerve of the contralateral uninjected eye points towards a possible diffusion pathway. Further experimental work will clarify these interesting findings.”
“We are excited about these scientifically significant results,” commented Bernard Gilly, Co-founder and Chief Executive Officer of GenSight. “Moreover, they vindicate the company’s position that the unexpected bilateral improvements seen in the REVERSE and RESCUE trials have a solid scientific basis. The results help provide a compelling argument in support of GS010’s marketing authorization application.”
GenSight is working with its panel of scientific experts to prepare the findings for submission to a peer-reviewed journal later this year.
Dr. Yu-Wai-Man will discuss these findings when he presents RESCUE results at the 2019 annual meeting of the American Academy of Ophthalmology in San Francisco, CA:
- Session Date: Sunday, October 13
- Paper Session: OP04 Neuro-Ophthalmology Original Paper
- Session Time: 2:00 PM to 3:15 PM
- Location: South 152
- Presenter: Patrick Yu-Wai-Man, FRCOphth MBBS PhD
- Presentation time: 3:00 p.m.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe.
About REVERSE and RESCUE
REVERSE and RESCUE are two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best‑Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best‑seeing eyes that received GS010 compared to those receiving sham, and to worse‑seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio‑dissemination and the time course of immune response. Readouts for these endpoints are at 48, 72 and 96 weeks after injection.
The trials are conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results were reported in 2019 for both trials, after which patients were transferred to a long-term follow-up study that will last for three years.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation.
The trial planned to enroll 90 patients with vision loss up to 1 year in duration and will be conducted in multiple centers in Europe and in the US.
In the active arm, GS010 will be administered as a single intravitreal injection to both eyes of each subject. In the placebo arm, GS010 will be administered as a single intravitreal injection to the first affected eye, while the fellow eye will receive a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1-Year post-treatment in the second‑affected/not‑yet‑affected eye. The change from baseline in second‑affected/not‑yet‑affected eyes receiving GS010 and placebo will be the primary response of interest. The secondary efficacy endpoints include: BCVA reported in LogMAR at 2-Years post-treatment in the second‑affected/not‑yet‑affected eye compared to both placebo and the first‑affected eye receiving GS010, OCT and contrast sensitivity and quality of life scales. The first subject was treated in March 2018, and enrolment was completed in July 2019, ahead of schedule.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524