Paris, France, Wednesday, July 20, 7.30 am CEST – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported that after 5 years of follow-up, Leber Hereditary Optical Neuropathy (LHON) subjects treated with LUMEVOQ® (GS010) continued to experience significantly improved vision as a result of a one-time injection of the gene therapy treatment. Compared to the trend in vision observed among untreated patients1, the findings are a significant divergence from the natural outcomes of LHON.
The data from RESTORE (CLIN06)2, the long-term follow-up study to which all participants in the RESCUE3 and REVERSE4 Phase III pivotal trials were invited, also continue to show that the treatment is well-tolerated over the 5-year follow-up period.
5 years’ data on efficacy and safety shows substantial durability evidence and is more extensive than what is typically submitted in a data package for a gene therapy.
When RESTORE subjects enrolled in the study 2 years after the one-time injection, they had already experienced clinically meaningful improvements relative to the lowest point (the “nadir”) of their best-corrected visual acuity (BCVA): +18.8 ETDRS letters equivalent* in their LUMEVOQ®-treated eyes and +17.3 letters equivalent in their sham-treated eyes. 5 years after treatment, the bilateral improvement from nadir was sustained, with LUMEVOQ®-treated eyes achieving a mean improvement against nadir of +22.0 letters equivalent and sham-treated eyes demonstrating a mean improvement of +19.5 letters equivalent.
The impact of such results on patients is demonstrated by increases in the self-reported quality of life (QoL) scores at Year 5 vs. baseline. Mean overall QoL increased by a clinically meaningful magnitude relative to baseline, driven by increases in the sub-scores corresponding to mental health and the ability to carry out activities autonomously (e.g., composite score, mental health, role difficulties, dependency, near and distance activities, general vision, social functioning).
“The 5-year data from the RESTORE long-term extension study illustrates that the efficacy and safety data previously reported following treatment with a one-time injection of LUMEVOQ® is being maintained, providing hope to patients affected with this debilitating blinding disease” said Patrick Yu-Wai-Man, MD, PhD, Professor of Ophthalmology and Honorary Consultant Neuro-ophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, United Kingdom.
“The data so far showing the sustained efficacy of LUMEVOQ®, combined with a favorable safety profile, is fully consistent with clinical experts’ belief that the gene therapy’s effects will not wane after its administration” noted Magali TAIEL, MD, Chief Medical Officer of GenSight Biologics. “With even more confidence about the benefit we can deliver to patients, the GenSight team is working with full intensity to bring our therapy to patients as quickly and safely as possible.”
RESTORE is a large long-term follow-up study for a rare disease treatment, with 62 subjects accepting the invitation to enroll and 55 completing the study. All subjects, who were affected by LHON caused by a mutated ND4 mitochondrial gene, were treated with an intravitreal injection of LUMEVOQ® in one eye and with sham injection in the other.
Table 1. BCVA Mean Improvement Vs. Nadir* In LUMEVOQ® Long-Term Follow-Up (RESTORE)
| 2 Years Post-Injection
(Start of RESTORE) |
5 Years Post-Injection4
(End of RESTORE) |
|||
| LogMAR
(Std Error) |
Letters Equivalent** | LogMAR
(Std Error) |
Letters Equivalent** | |
| LUMEVOQ®-treated eyes | -0.38
(0.31) |
+18.8
|
-0.44
(0.46) |
+22.0
|
| Sham-treated eyes | -0.35
(0.29) |
+17.3
|
-0.39
(0.36) |
+19.5
|
Note: The RESTORE sample consists of the RESCUE and REVERSE participants who accepted to be followed in the long-term follow-up study. Year 5 values were the LogMAR readings nearest to 1,825 days post treatment recorded between 1,825 +/- 28 days post-treatment. Missing values were imputed using the Last Observation Carried Forward (LOCF) method.
*Nadir = worst best-corrected visual acuity recorded from baseline to Year 5.
** Assessments of best-corrected visual acuity (BCVA) were recorded in LogMAR. The change from nadir in LogMAR was converted to “letters equivalent” improvement by multiplying the LogMAR by -50 (ref. J.T. Holladay, J Refrac Surgery, 1997;13, 388-391).
Responder analyses at Year 5 indicate that improved BCVA was a benefit for a substantial proportion of the study participants. 71.0% of RESTORE subjects achieved Clinically Relevant Recovery (CRR)5 against nadir 5 years after treatment, and 80.7% of them had on-chart vision (BCVA ≤ 1.6 LogMAR) in one or both eyes.
Figure 1. Evolution of BCVA In LUMEVOQ®-treated Patients (RESCUE/REVERSE/RESTORE) vs. Untreated Patients
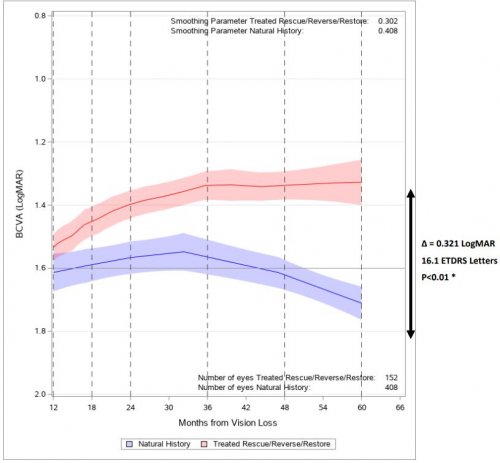
Note: The Locally Estimated Scatterplot Smoothing (LOESS) curves show the evolution of BCVA, from 12 months to 60 months after onset of vision loss, in all eyes (LUMEVOQ®– and sham-treated) from REVERSE / RESCUE / RESTORE studies and all eyes from a matched cohort of patients not treated with LUMEVOQ®. The shaded areas represent the 95% confidence interval for the BCVA values. The values >60 months were set to 60 months. The curve starts at 12 months after onset when 92.7% of eyes in RESCUE and REVERSE had received treatment, either with LUMEVOQ® or a sham injection. The untreated cohort consisted of 208 ND4-LHON patients that were followed in the REALITY registry and from two prospective and eight retrospective natural history studies.6
* p<0.01 for Kruskal-Wallis test and Repeated measures on patient.
Safety findings at 5 years post-injection were consistent with previous readouts, which concluded that LUMEVOQ® is well-tolerated: no serious adverse events were recorded among LUMEVOQ®-treated eyes, and no discontinuations occurred due to ocular events. There were no systemic serious adverse events or discontinuations related to study treatment or study procedure.
The review of the European Marketing Authorisation Application for LUMEVOQ® is ongoing, with the decision from the CHMP expected in Q3 2023 as a result of an extension granted by the EMA for GenSight’s responses to its Day 120 questions. Commercial launch will follow approval by the end of 2023.
References and notes:
- Newman NJ, Yu-Wai-Man P, Carelli V, et al.,. Intravitreal Gene Therapy vs. Natural History in Patients With Leber Hereditary Optic Neuropathy Carrying the m.11778G>A ND4 Mutation: Systematic Review and Indirect Comparison. Neurol. (2021) 12:662838. doi: 10.3389/fneur.2021.662838.
- Biousse, V, Newman, N, Yu-Wai-Man P, et al. Long-Term Follow-Up After Unilateral Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy: The RESTORE Study, J Neuroophthalmol. (2021) 41: 309-315. doi: 10.1097/WNO.0000000000001367.
- Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology (2021) 128:649–60. doi: 10.1016/j.ophtha.2020.12.012.
- Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. (2020) 12:eaaz7423. doi: 10.1126/scitranslmed.aaz7423.
- Clinically Relevant Recovery (CRR) corresponds to an improvement of at least 0.2 LogMAR (for on-chart eyes) or a movement from off-chart to on-chart (for off-chart eyes).
- Newman NJ, Yu-Wai-Man P, Carelli V, et al. Intravitreal gene therapy vs. natural history in patients with Leber hereditary optic neuropathy carrying the m.11778G>A ND4 mutation: systematic review and indirect comparison. Front Neurol. 2021;12:662838.
Contacts
-
LifeSci AdvisorsInvestor RelationsGuillaume van Renterghem+41 (0)76 735 01 31