- Confirmed clinically significant and sustained improvement in visual acuity of 174 patients with Leber Hereditary Optic Neuropathy carrying the ND4 mutation (ND4-LHON) treated with LUMEVOQ®
- 90% of eyes treated with LUMEVOQ® on-chart 4 years after their vision loss
- LUMEVOQ® continues to show promise as a novel therapy for the treatment of ND4-LHON
Paris, France, December 15, 2022, 7:30 am CET – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced the publication of a peer-reviewed article in the journal Ophthalmology and Therapy highlighting updated efficacy results from a pooled analysis of four Phase 3 studies showing an improvement in visual acuity in ND4-LHON patients treated with lenadogene nolparvovec (LUMEVOQ®).
The article, entitled “Indirect Comparison of Lenadogene Nolparvovec Gene Therapy Versus Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A MT-ND4 Mutation”, incorporates data from the latest Phase 3 trial REFLECT, increasing the number of treated patients from 76 to 174 since the previously published pooled analysis. A group of 208 matched patients from natural history studies was used as an external control group.
The inclusion of REFLECT data permits outcomes in bilaterally treated eyes to be compared to those of patients treated unilaterally. When adjusted for covariates, the bilateral intravitreal injection (IVT) data presented in the article showed an improvement of +22.5 ETDRS letters versus natural history, as compared to an improvement of +17.5 ETDRS letters versus natural history for the unilateral IVT. Bilateral IVT also had an on-chart response rate of 79.2% compared to 67.0% for those with the unilateral IVT.
Overall, the patients with LUMEVOQ® showed a clinically significant and sustained improvement in their visual acuity when compared to the natural history patients. Mean improvement versus natural history was +15 ETDRS letters up to 3.9 years after treatment (p<0.01). At 4 years (48 months) after vision loss, the majority of treated eyes were on-chart compared to less than half of natural history eyes (89.6% versus 48.1%) (p<0.01). When adjusted for covariates of interest (gender, age of onset, ethnicity, and duration of follow-up), the estimated mean gain was – 0.43 logMAR (+ 21.5 ETDRS letters equivalent) versus natural history at last observation (p<0.0001). Thus, the treatment effect remained highly clinically significant when controlling for potential confounding factors (figure 1).
The evolution of natural history eyes showed an absence of recovery throughout the entire follow-up period, with a plateau up to 36 months followed by a slow decline. By contrast, eyes treated with LUMEVOQ® showed a progressive, continuous and sustained improvement between 12 and 52 months after vision loss.
“The results published in this peer-reviewed scientific paper provide more evidence of LUMEVOQ’s potential as an effective treatment for LHON. The results indicate that LUMEVOQ treatment offers a better chance of recovery of vision than the published natural history of this disorder, giving hope to people affected by this debilitating blinding disease.” said Valerio Carelli, MD, PhD, Professor of Medical Genetics, Director of the Program in Neurogenetics, Department of Biomedical and NeuroMotor Sciences, University of Bologna School of Medicine and lead author of the article.
The data was taken from four pooled Phase 3 studies, REVERSE, RESCUE and their long-term extension trial RESTORE, as well as the REFLECT trial. In the first three trials LUMEVOQâ was administered exclusively as a unilateral intravitreal injection (IVT), while in the REFLECT trial it was assessed as either an unilateral or a bilateral IVT.
The article is available in print and online via this link.
Figure 1: from Indirect Comparison of Lenadogene Nolparvovec Gene Therapy Versus Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A MT-ND4 Mutation
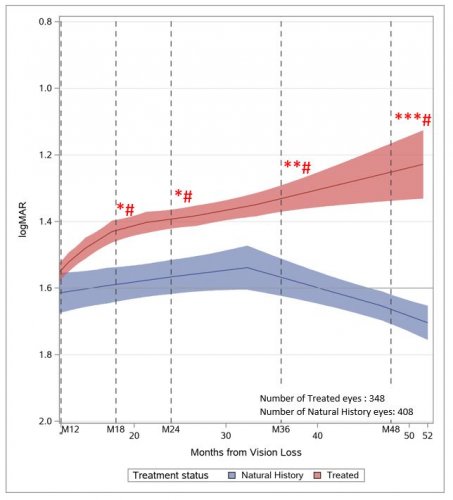
Note: Evolution of visual acuity of treated eyes versus natural history eyes. The evolution of visual acuities over time for treated eyes (n = 348) and natural history eyes (n = 408) was estimated by LOESS regression (solid line) with 95% CI around the fitted curve (shaded area). Visual acuity values > 52 months were assigned to the 52-month time point using the next observation carried backward method. Smoothing parameter: 0.315 for treated eyes and 0.408 for natural history eyes. The statistically significant difference between treated and natural history eyes is illustrated by the non-overlapping CIs of LOESS curves. Mean differences at month 18 [15; 21], month 24 [21; 30], month 36 [30; 42] and month 48 [42; 54] were estimated by a mixed-model ANCOVA with repeated measures: *P = 0.03, **P = 0.02 and ***P < 0.01 versus natural history; and with Kruskal Wallis test: #P < 0.01 versus natural history.
Contacts
-
GenSight BiologicsCorporate Communications DirectorClothilde Caillet
-
LifeSci AdvisorsInvestor RelationsGuillaume van Renterghem+41 (0)76 735 01 31